Titanium Breast Marker Lawsuit
Patients, medical professionals, manufacturers, and legal representatives are embroiled in a dispute over titanium breast marker clips. The crux of the lawsuit is the failure to warn about the potential adverse reactions to these clips used in breast cancer treatment.
Patients who experienced complications allege that they weren’t adequately informed about the potential risks associated with these marker clips. This lack of informed consent is at the heart of their grievances.
The relief being sought is multi-faceted. Here are the top 3 requests:
- Financial compensation for medical expenses incurred due to complications from the titanium marker clips.
- Reimbursement for the pain and suffering endured by the patients.
- Removal of the titanium markers is a procedure that may require additional surgery and, thus, more expenses.
In terms of the lawsuit’s current status, it’s still in the ongoing legal proceedings stage.
Titanium Breast Marker Lawsuit Explanation
The heart of these lawsuits revolves around complications from titanium-based markers used in breast cancer treatment.
As a breast cancer patient, you might’ve experienced adverse reactions from titanium marker clips left in your breast tissue. Complaints range from pain and inflammation to skin sensitivity and discomfort. These aren’t minor inconveniences; they can impact your daily life and overall well-being.
The lawsuits argue that manufacturers failed to adequately warn about the risks of these titanium markers. You may not have known about the potential for non-absorption, protrusion, scarring, and asymmetry. These complications can lead to further medical treatments, emotional distress, and even financial strain.
If you’ve suffered because of these titanium marker clips, you might be entitled to compensation. Lawsuits aim to recover medical expenses, pain and suffering, lost wages, and other related damages.
Parties involved
- You, the patient: You’ve undergone treatment involving a titanium marker. But something’s gone wrong, causing you physical or emotional distress.
- The medical facility: This is the place where your treatment was carried out. They must ensure the safety of the procedures they perform.
- The manufacturer: They produced the titanium marker used in your treatment. If they fail to warn about potential risks or are negligent, they share responsibility.
The cause of action
In a titanium breast marker lawsuit, the cause of action involves allegations that manufacturers failed to adequately warn about potential risks associated with the implant. You might claim that the maker of the device, such as Hologic, didn’t adequately inform you of the non-absorption, palpable masses, pain, and other side effects of these marker clips.
The crux of your lawsuit would be the complications you experienced after the implant. This could include discomfort, protrusion, asymmetry, and even the need for additional radiation therapy. These complications can cause significant pain, leading to a decrease in your quality of life.
A key point in your lawsuit will be the lack of informed consent. You may argue that you weren’t properly informed about the risks and potential complications associated with this device.
Relief being sought
Seeking relief, you may file a titanium breast marker lawsuit to obtain compensation for medical expenses, pain, suffering, lost wages, and other related damages caused by complications from the device. The goal of such legal action is to secure a financial recovery for any adverse effects you’ve suffered.
When pursuing this lawsuit, it’s crucial to understand what you’re entitled to. Here are three main areas of relief you may seek:
- Compensation: This includes covering medical expenses incurred due to complications, compensating for pain and suffering, and making up for lost wages. The amount varies based on the extent of your losses and damages.
- Acknowledgment of Risks: You’re seeking acknowledgement from manufacturers, like Hologic, regarding the risks associated with titanium breast markers and proper warnings about these risks.
- Legal Assistance: Given the complexities of such cases, you’ll need legal assistance in navigating the lawsuit and pursuing your rightful claims.
Key events and timeline
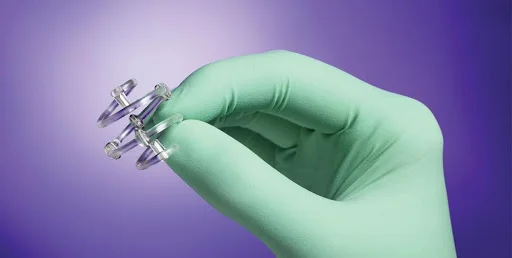
The healthcare industry introduced titanium markers, specifically BioZorb implants, as a revolutionary tool in breast cancer treatment. However, shortly after their introduction, patients began reporting allergic reactions to titanium. These reactions included pain, inflammation, and skin rashes, which caused significant discomfort.
As these cases increased, some patients had to undergo additional surgeries for the removal of these titanium markers. This situation led to a series of lawsuits against the manufacturers of these markers. The basis of these lawsuits was the lack of adequate information given to patients about the potential risks of BioZorb and other titanium-based marker clips.
Key arguments
Here are the main arguments:
- Lack of Adequate Warning: Case reports suggest that manufacturers failed in their duty to inform patients about the risks involved with the use of titanium breast markers. This is especially relevant in scenarios involving BioZorb, a specific brand of these markers.
- Adverse Reactions: Many patients have experienced pain, discomfort, inflammation, and skin sensitivity after surgery. These adverse reactions highlight the need for thorough patient education and informed consent.
- Compensation: Patients are seeking compensation for their suffering, arguing that they weren’t adequately warned. Legal experts can assist in evaluating eligibility for compensation.
Current status
The current status of these lawsuits is ongoing, with legal experts diligently working on behalf of their clients. They’re gathering evidence, documenting the extent of the complications, and fighting for fair compensation for their clients.
Implications
- Health Implications: As a patient, you might experience complications such as pain, inflammation, swelling, and skin reactions due to adverse reactions to the titanium marker clips. In some cases, these complications can escalate leading to discomfort, granulomatous reactions, and persistent pain. Particularly, if you’re diagnosed with DCIS, you could face complications from titanium clips left in your breast tissue.
- Hypersensitivity Issues: Hypersensitivity to titanium is another concern. Allergic reactions, contact dermatitis, and eczematiforme column are some documented cases of hypersensitivity to titanium in medical contexts.
- Legal Implications: Legal issues have arisen regarding the lack of informed consent. This means that you, as a patient, mightn’t have been fully informed about the potential risks and adverse events associated with titanium-based markers.

Business Manager